1. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506–14.
2. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995 Jun;125(6):1401–12.
3. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020 Nov;17(11):687–701.
4. Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014 Aug;32(8):834–41.
5. Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56(6):680–6.
6. Collinson S, Deans A, Padua-Zamora A, Gregorio GV, Li C, Dans LF, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD003048.
7. Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019 Apr 30;4(4):CD004827.
8. Goodman C, Keating G, Georgousopoulou E, Hespe C, Levett K. Probiotics for the prevention of antibiotic-associated diarrhoea: a systematic review and meta-analysis. BMJ Open. 2021 Aug 12;11(8):e043054.
9. Zhang L, Zeng X, Guo D, Zou Y, Gan H, Huang X. Early use of probiotics might prevent antibiotic-associated diarrhea in elderly (>65 years): a systematic review and meta-analysis. BMC Geriatr. 2022 Jul 6;22(1):562.
10. Goldenberg JZ, Yap C, Lytvyn L, Lo CKF, Beardsley J, Mertz D, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017 Dec 19;12(12):CD006095.
11. Hamad A, Fragkos KC, Forbes A. A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013 Jun;32(3):353–60.
12. Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022 Aug 8;gutjnl-2022-327745.
13. Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017 Feb 23;2(2):CD008716.
14. Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV, et al. AGA Clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020 Aug;159(2):697–705.
15. Kaur L, Gordon M, Baines PA, Iheozor-Ejiofor Z, Sinopoulou V, Akobeng AK. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020 Mar 4;3(3):CD005573.
16. Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk, and health: a systematic review. Nutr Rev. 2021 Apr 7;79(5):599–614.
17. van den Akker CHP, van Goudoever JB, Shamir R, Domellöf M, Embleton ND, Hojsak I, et al. Probiotics and preterm infants: a position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2020 May;70(5):664–80.
18. Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2020 Oct 15;10(10):CD005496.
19. Zhang GQ, Hu HJ, Liu CY, Zhang Q, Shakya S, Li ZY. Probiotics for prevention of atopy and food hypersensitivity in early childhood: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016 Feb;95(8):e2562.
20. Hatakka K, Ahola AJ, Yli-Knuuttila H, Richardson M, Poussa T, Meurman JH, et al. Probiotics reduce the prevalence of oral candida in the elderly—a randomized controlled trial. J Dent Res. 2007 Feb;86(2):125–30.
21. Kraft-Bodi E, Jørgensen MR, Keller MK, Kragelund C, Twetman S. Effect of probiotic bacteria on oral candida in frail elderly. J Dent Res. 2015 Sep;94(9 Suppl):181S-6S.
22. Ishikawa KH, Mayer MPA, Miyazima TY, Matsubara VH, Silva EG, Paula CR, et al. A Multispecies probiotic reduces oral Candida colonization in denture wearers: reduction of Candida by probiotics. J Prosthodont. 2015 Apr;24(3):194–9.
23. Grossi E, Buresta R, Abbiati R, Cerutti R. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with acute diarrhea: a multicenter, randomized study in primary care. J Clin Gastroenterol. 2010 Sep;44(Supplement 1):S35–41.
24. McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010 May 14;16(18):2202–22.
25. Greuter T, Michel MC, Thomann D, Weigmann H, Vavricka SR. Randomized, placebo-controlled, double-blind and open-label studies in the treatment and prevention of acute diarrhea with Enterococcus faecium SF68. Front Med. 2020;7:276.
26. Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JNV, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012 May 9;307(18):1959–69.
27. Liao W, Chen C, Wen T, Zhao Q. Probiotics for the prevention of antibiotic-associated diarrhea in adults: a meta-analysis of randomized placebo-controlled trials. J Clin Gastroenterol. 2021 Jul 1;55(6):469–80.
28. Cai J, Zhao C, Du Y, Zhang Y, Zhao M, Zhao Q. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: Systematic review with network meta-analysis. United Eur Gastroenterol J. 2018 Mar;6(2):169–80.
29. Szajewska H, KoÅ‚odziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015 Oct;42(7):793–801.
30. Cimperman L, Bayless G, Best K, Diligente A, Mordarski B, Oster M, et al. A randomized, double-blind, placebo-controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol. 2011 Oct;45(9):785–9.
31. Ouwehand AC, DongLian C, Weijian X, Stewart M, Ni J, Stewart T, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014 Jan 16;32(4):458–63.
32. Koning CJM, Jonkers DMAE, Stobberingh EE, Mulder L, Rombouts FM, Stockbrügger RW. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am J Gastroenterol. 2008 Jan;103(1):178–89.
33. Wenus C, Goll R, Loken EB, Biong AS, Halvorsen DS, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008 Feb;62(2):299–301.
34. Selinger CP, Bell A, Cairns A, Lockett M, Sebastian S, Haslam N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J Hosp Infect. 2013 Jun;84(2):159–65.
35. Johnson S, Maziade PJ, McFarland LV, Trick W, Donskey C, Currie B, et al. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int J Infect Dis. 2012 Nov;16(11):e786-792.
36. Shen NT, Maw A, Tmanova LL, Pino A, Ancy K, Crawford CV, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017 Jun;152(8):1889-1900.e9.
37. Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004 Mar;7(1):59–62.
38. Lewis S, Burmeister S, Brazier J. Effect of the prebiotic oligofructose on relapse of Clostridium difficile-associated diarrhea: a randomized, controlled study. Clin Gastroenterol Hepatol. 2005 May;3(5):442–8.
39. Yu M, Zhang R, Ni P, Chen S, Duan G. Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: a meta-analysis of randomized controlled trials. PloS One. 2019;14(10):e0223309.
40. Hauser G, Salkic N, Vukelic K, JajacKnez A, Stimac D. Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial. Medicine (Baltimore). 2015 May;94(17):e685.
41. Seddik H, Boutallaka H, Elkoti I, Nejjari F, Berraida R, Berrag S, et al. Saccharomyces boulardii CNCM I-745 plus sequential therapy for Helicobacter pylori infections: a randomized, open-label trial. Eur J Clin Pharmacol. 2019 May;75(5):639–45.
42. Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004 Nov 15;20(10):1181–8.
43. Plomer M, III Perez M, Greifenberg DM. Effect of Bacillus clausii capsules in reducing adverse effects associated with Helicobacter pylori eradication therapy: a randomized, double-blind, controlled trial. Infect Dis Ther. 2020 Dec;9(4):867–78.
44. Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori. J Med Food. 2011 Apr;14(4):344–7.
45. Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007 Feb 14;13(6):912–5.
46. Liu MM, Li ST, Shu Y, Zhan HQ. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PloS One. 2017;12(6):e0178870.
47. Wei D, Heus P, van de Wetering FT, van Tienhoven G, Verleye L, Scholten RJ. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst Rev. 2018 Aug 31;8(8):CD008831.
48. Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010 May 5;5:31.
49. Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014 Oct;33(5):761–7.
50. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019 Jun;11(2):638–47.
51. Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Rao Z, et al. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: a prospective randomized and controlled trial. Medicine (Baltimore). 2017 Oct;96(43):e8418.
52. de Castro Soares GG, Marinho CH, Pitol R, Andretta C, Oliveira E, Martins C, et al. Sporulated Bacillus as alternative treatment for diarrhea of hospitalized adult patients under enteral nutrition: a pilot randomized controlled study. Clin Nutr ESPEN. 2017 Dec;22:13–8.
53. Frohmader TJ, Chaboyer WP, Robertson IK, Gowardman J. Decrease in frequency of liquid stool in enterally fed critically ill patients given the multispecies probiotic VSL#3: a pilot trial. Am J Crit Care. 2010 May 1;19(3):e1–11.
54. Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016 May 6;2016(5):CD003044.
55. Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014 Jun;12(6):1003-1008.e1.
56. Dhiman RK, Thumburu KK, Verma N, Chopra M, Rathi S, Dutta U, et al. Comparative efficacy of treatment options for minimal hepatic encephalopathy: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2020 Apr;18(4):800-812.e25.
57. Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2011 Aug;23(8):725–32.
58. Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008 Jul;103(7):1707–15.
59. Ziada DH, Soliman HH, El Yamany SA, Hamisa MF, Hasan AM. Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? A neurometabolite study using magnetic resonance spectroscopy. Arab J Gastroenterol. 2013 Sep;14(3):116–22.
60. Vlachogiannakos J, Vasianopoulou P, Viazis N, Chroni M, Voulgaris T, Ladas S, et al. The role of probiotics in the treatment of minimal hepatic encephalopathy. A prospective, randomized, placebo-controlled, double-blind study [abstract]. Hepatology. 2014;60((4 Suppl)):376A.
61. Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014 Dec;97(12):7386–93.
62. Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014 Mar;99(3):535–42.
63. Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017 Mar;117(5):662–8.
64. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012 Feb;57(2):545–53.
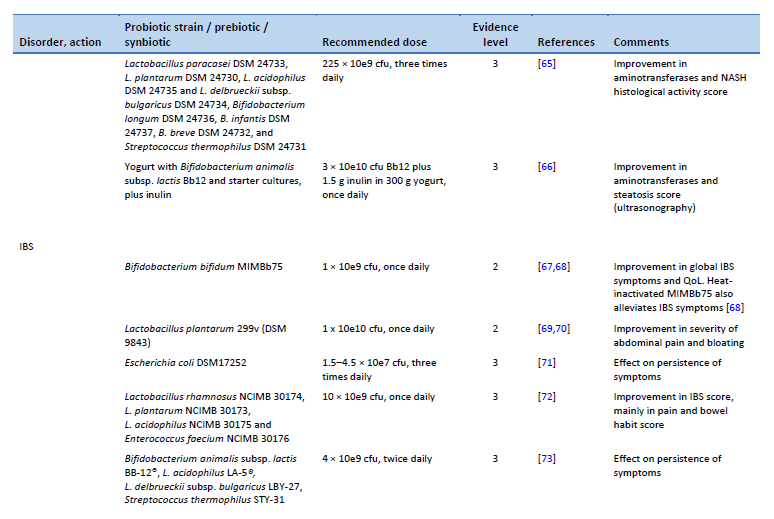
65. Duseja A, Acharya SK, Mehta M, Chhabra S, Shalimar, Rana S, et al. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019 Aug;6(1):e000315.
66. Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr. 2018 Aug 1;148(8):1276–84.
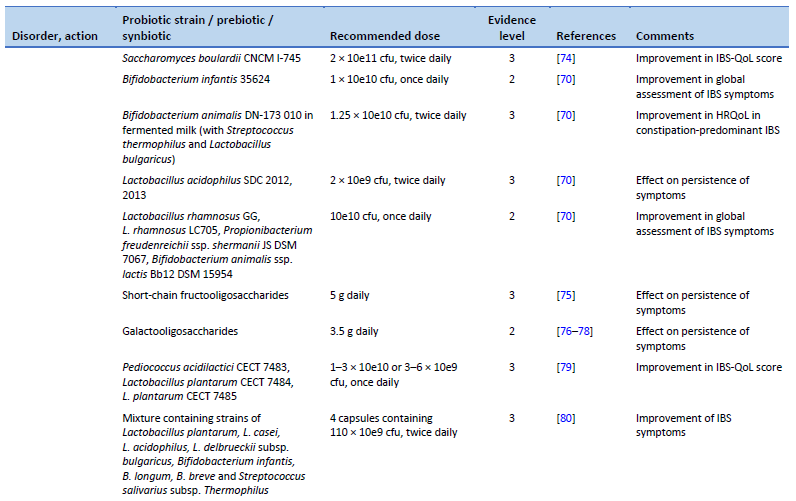
67. Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011 May;33(10):1123–32.
68. Andresen V, Gschossmann J, Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol Hepatol. 2020 Jul;5(7):658–66.
69. Ducrotté P. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18(30):4012.
70. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018 Nov;48(10):1044–60.
71. Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009 Feb;47(2):209–14.
72. Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome--a 12 week double-blind study. Aliment Pharmacol Ther. 2014 Jul;40(1):51–62.
73. Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014 Jul;17(7):466–70.
74. Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011 Sep;45(8):679–83.
75. Paineau D, Payen F, Panserieu S, Coulombier G, Sobaszek A, Lartigau I, et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr. 2008 Feb;99(2):311–8.
76. Silk DBA, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009 Mar;29(5):508–18.
77. Vulevic J, Tzortzis G, Juric A, Gibson GR. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol Motil. 2018 Nov;30(11):e13440.
78. Huaman JW, Mego M, Manichanh C, Cañellas N, Cañueto D, Segurola H, et al. Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology. 2018 Oct;155(4):1004–7.
79. Lorenzo-Zúñiga V, Llop E, Suárez C, Alvarez B, Abreu L, Espadaler J, et al. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol. 2014 Jul 14;20(26):8709–16.
80. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015 Jan;60(1):186–94.
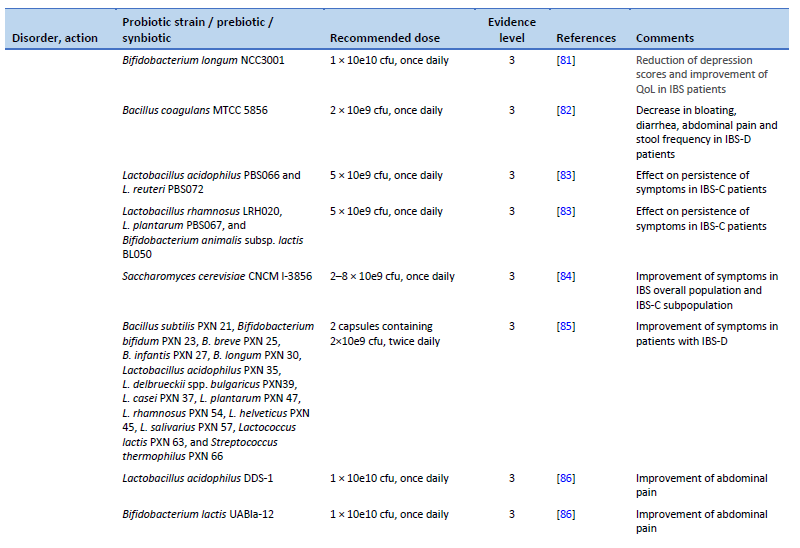
81. Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017 Aug;153(2):448-459.e8.
82. Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, et al. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J. 2015 Dec;15(1):21.
83. Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, La Ferla B, Schiano I, et al. A randomized, double-blind, placebo-controlled trial: the efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. BioMed Res Int. 2016;2016:4740907.
84. Cayzeele-Decherf A, Pélerin F, Leuillet S, Douillard B, Housez B, Cazaubiel M, et al. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: an individual subject meta-analysis. World J Gastroenterol. 2017;23(2):336.
85. Ishaque SM, Khosruzzaman SM, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018 May 25;18(1):71.
86. Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 improve abdominal pain severity and symptomology in irritable bowel syndrome: randomized controlled trial. Nutrients. 2020 Jan 30;12(2):363.
87. Sadrin S, Sennoune S, Gout B, Marque S, Moreau J, Zinoune K, et al. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: a placebo-controlled randomized clinical trial. Dig Liver Dis. 2020 May;52(5):534–40.
88. Francavilla R, Piccolo M, Francavilla A, Polimeno L, Semeraro F, Cristofori F, et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Gastroenterol. 2019 Mar;53(3):e117–25.
89. Smecuol E, Constante M, Temprano MP, Costa AF, Moreno ML, Pinto-Sanchez MI, et al. Effect of Bifidobacterium infantis NLS super strain in symptomatic coeliac disease patients on long-term gluten-free diet—an exploratory study. Benef Microbes. 2020 Oct 12;11(6):527–34.
90. Yeun Y, Lee J. Effect of a double-coated probiotic formulation on functional constipation in the elderly: a randomized, double blind, controlled study. Arch Pharm Res. 2015 Jul;38(7):1345–50.
91. Ojetti V, Ianiro G, Tortora A, D‘Angelo G, Di Rienzo TA, Bibbò S, et al. The effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation: a randomized, double-blind, placebo-controlled trial. J Gastrointestin Liver Dis. 2014 Dec 1;23(4):387–91.
92. Riezzo G, Orlando A, D’Attoma B, Linsalata M, Martulli M, Russo F. Randomised double blind placebo controlled trial on Lactobacillus reuteri DSM 17938: improvement in symptoms and bowel habit in functional constipation. Benef Microbes. 2018 Jan 29;9(1):51–60.
93. Schumann C. Medical, nutritional and technological properties of lactulose. An update. Eur J Nutr. 2002;41(Suppl 1):I17-25.
94. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2015;13(1):3951.
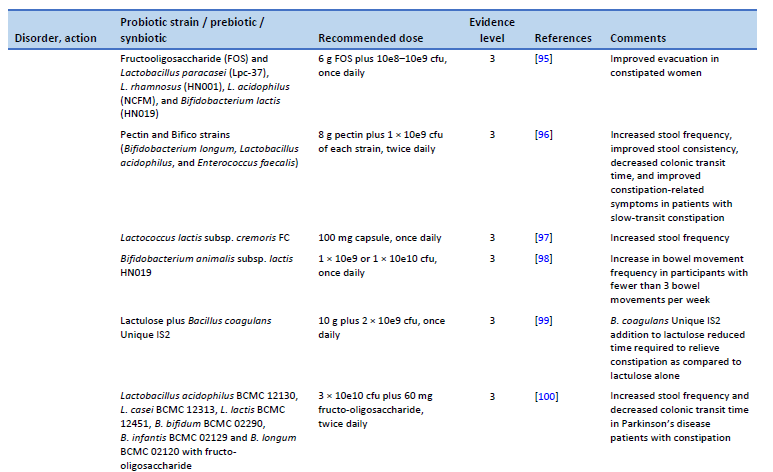
95. Waitzberg DL, Logullo LC, Bittencourt AF, Torrinhas RS, Shiroma GM, Paulino NP, et al. Effect of synbiotic in constipated adult women – a randomized, double-blind, placebo-controlled study of clinical response. Clin Nutr. 2013 Feb;32(1):27–33.
96. Ding C, Ge X, Zhang X, Tian H, Wang H, Gu L, et al. Efficacy of synbiotics in patients with slow transit constipation: a prospective randomized trial. Nutrients. 2016 Sep 28;8(10):605.
97. Toda T, Nanba F, Arai K, Takamizawa N, Shioya N, Suzuki S. Effect of supplement containing Lactococcus lactis subsp. cremoris FC on defecation in healthy humans: a randomized, placebo-controlled, double-blind crossover trial. Jpn Pharmacol Ther. 2017;45(6):989–97.
98. Ibarra A, Latreille-Barbier M, Donazzolo Y, Pelletier X, Ouwehand AC. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018 May 4;9(3):236–51.
99. Venkataraman R, Shenoy R, Ahire JJ, Neelamraju J, Madempudi RS. Effect of Bacillus coagulans unique IS2 with lactulose on functional constipation in adults: a double-blind placebo controlled study. Probiotics Antimicrob Proteins [Internet]. 2021 Oct 2 [cited 2023 Feb 22]; Available from: https://link.springer.com/10.1007/s12602-021-09855-8
100. Ibrahim A, Ali RAR, Manaf MRA, Ahmad N, Tajurruddin FW, Qin WZ, et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: a randomised controlled trial. Plos One. 2020 Dec 31;15(12):e0244680.
101. Sakai T, Makino H, Ishikawa E, Oishi K, Kushiro A. Fermented milk containing Lactobacillus casei strain Shirota reduces incidence of hard or lumpy stools in healthy population. Int J Food Sci Nutr. 2011 Jun;62(4):423–30.
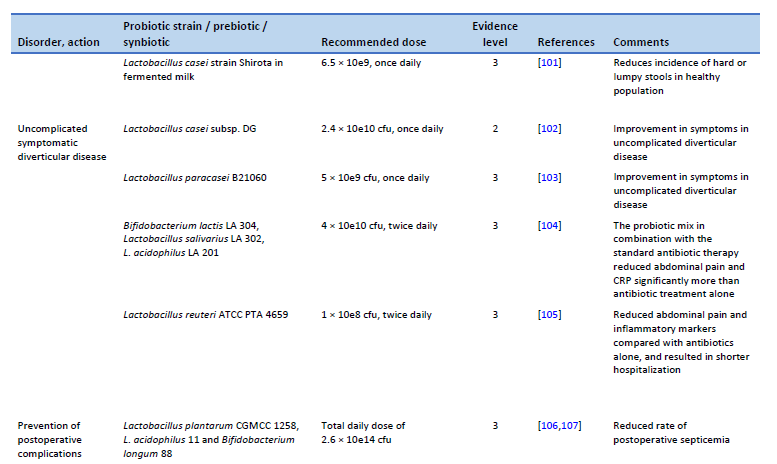
102. Tursi A, Brandimarte G, Elisei W, Picchio M, Forti G, Pianese G, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease—a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther. 2013 Oct;38(7):741–51.
103. Lahner E, Esposito G, Zullo A, Hassan C, Cannaviello C, Paolo MCD, et al. High-fibre diet and Lactobacillus paracasei B21060 in symptomatic uncomplicated diverticular disease. World J Gastroenterol. 2012 Nov 7;18(41):5918–24.
104. Petruzziello C, Marannino M, Migneco A, Brigida M, Saviano A, Piccioni A, et al. The efficacy of a mix of three probiotic strains in reducing abdominal pain and inflammatory biomarkers in acute uncomplicated diverticulitis. Eur Rev Med Pharmacol Sci. 2019 Oct;23(20):9126–33.
105. Petruzziello C, Migneco A, Cardone S, Covino M, Saviano A, Franceschi F, et al. Supplementation with Lactobacillus reuteri ATCC PTA 4659 in patients affected by acute uncomplicated diverticulitis: a randomized double-blind placebo controlled trial. Int J Colorectal Dis. 2019 Jun;34(6):1087–94.
106. Liu Z, Li C, Huang M, Tong C, Zhang X, Wang L, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015 Mar 20;15:34.
107. Chowdhury AH, Adiamah A, Kushairi A, Varadhan KK, Krznaric Z, Kulkarni AD, et al. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2020 Jun;271(6):1036–47.
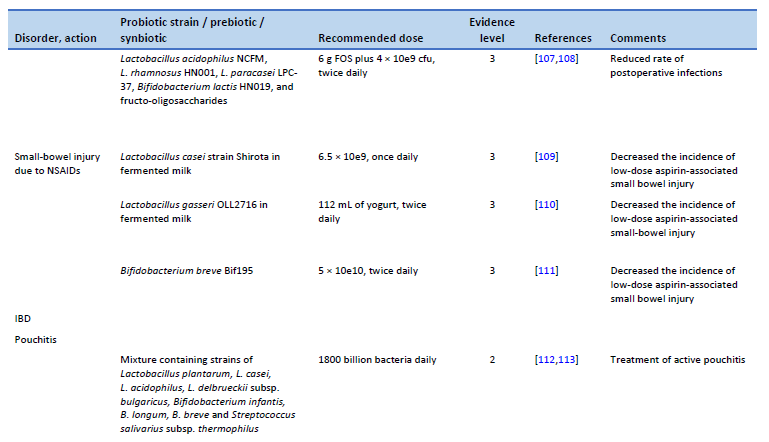
108. Flesch AT, Tonial ST, Contu PDC, Damin DC. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Rev Col Bras Cir. 2017;44(6):567–73.
109. Endo H, Higurashi T, Hosono K, Sakai E, Sekino Y, Iida H, et al. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. J Gastroenterol. 2011 Jul;46(7):894–905.
110. Suzuki T, Masui A, Nakamura J, Shiozawa H, Aoki J, Nakae H, et al. Yogurt containing Lactobacillus gasseri mitigates aspirin-induced small bowel injuries: a prospective, randomized, double-blind, placebo-controlled trial. Digestion. 2017;95(1):49–54.
111. Mortensen B, Murphy C, O’Grady J, Lucey M, Elsafi G, Barry L, et al. Bifidobacterium breve Bif195 protects against small-intestinal damage caused by acetylsalicylic acid in healthy volunteers. Gastroenterology. 2019 Sep;157(3):637-646.e4.
112. Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007 Dec;50(12):2075–82; discussion 2082–4.
113. Nguyen N, Zhang B, Holubar SD, Pardi DS, Singh S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2019 Nov 30;11(11):CD001176.
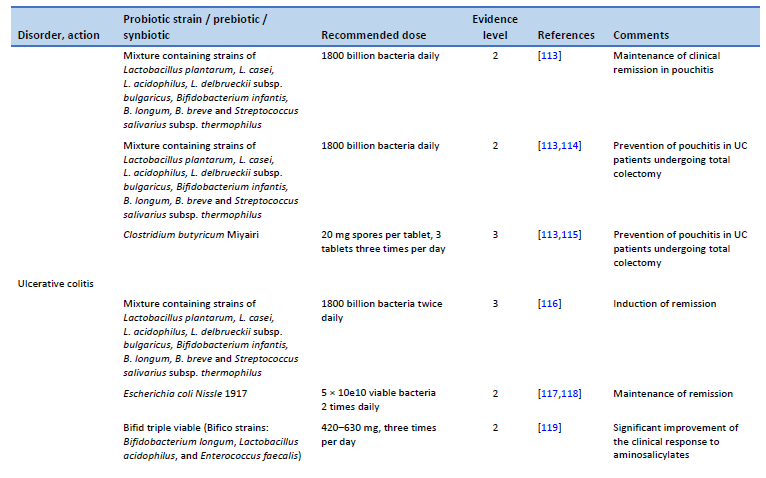
114. Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003 May;124(5):1202–9.
115. Yasueda A, Mizushima T, Nezu R, Sumi R, Tanaka M, Nishimura J, et al. The effect of Clostridium butyricum Miyairi on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today. 2016 Aug;46(8):939–49.
116. Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005 Jul;100(7):1539–46.
117. Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004 Nov;53(11):1617–23.
118. Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999 Aug 21;354(9179):635–9.
119. Chen MY, Qiu ZW, Tang HM, Zhuang KH, Cai QQ, Chen XL, et al. Efficacy and safety of bifid triple viable plus aminosalicylic acid for the treatment of ulcerative colitis: a systematic review and meta-analysis. Medicine (Baltimore). 2019 Nov;98(47):e17955.
120. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8(10):1763.
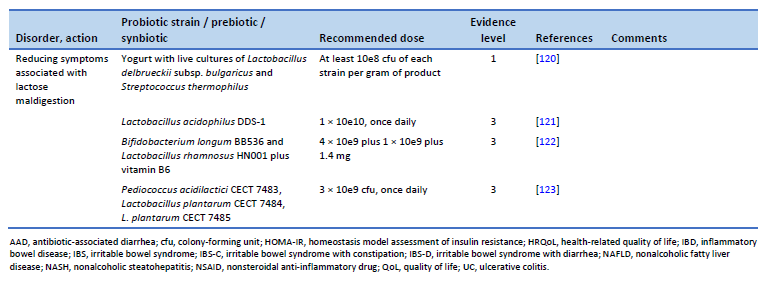
121. Pakdaman MN, Udani JK, Molina JP, Shahani M. The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance—a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr J. 2016 May 20;15(1):56.
122. Vitellio P, Celano G, Bonfrate L, Gobbetti M, Portincasa P, De Angelis M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms: a randomised, double-blind, cross-over study. Nutrients. 2019 Apr 19;11(4):886.
123. Cano-Contreras AD, Minero Alfaro IJ, Medina López VM, Amieva Balmori M, Remes Troche JM, Espadaler Mazo J, et al. Efficacy of i3.1 probiotic on improvement of lactose intolerance symptoms: a randomized, placebo-controlled clinical trial. J Clin Gastroenterol. 2022 Feb 1;56(2):141–7.
124. Szajewska H, Berni Canani R, Domellöf M, Guarino A, Hojsak I, Indrio F, et al. Probiotics for the management of pediatric gastrointestinal disorders: position paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J Pediatr Gastroenterol Nutr. 2023 Feb 1;76(2):232–47.
125. Szajewska H, KoÅ‚odziej M, Gieruszczak-BiaÅ‚ek D, Skórka A, RuszczyÅ„ski M, Shamir R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019 Jun;49(11):1376–84.
126. Szajewska H, KoÅ‚odziej M, Zalewski BM. Systematic review with meta-analysis: Saccharomyces boulardii for treating acute gastroenteritis in children—a 2020 update. Aliment Pharmacol Ther. 2020 Apr;51(7):678–88.
127. Patro-Gołąb B, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for treating acute gastroenteritis in children. An update. Nutrients. 2019 Nov 14;11(11):2762.
128. Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Møller PL, Tvede M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J. 2002 May;21(5):417–9.
129. Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Møller PL, Pedersen P, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J. 2002 May;21(5):411–6.
130. Ä°ÅŸlek A, Sayar E, Yılmaz A, Baysan BÖ, Mutlu D, Artan R. The role of Bifidobacterium lactis B94 plus inulin in the treatment of acute infectious diarrhea in children. Turk J Gastroenterol. 2014 Dec;25(6):628–33.
131. Passariello A, Terrin G, Cecere G, Micillo M, De Marco G, Di Costanzo M, et al. Randomised clinical trial: efficacy of a new synbiotic formulation containing Lactobacillus paracasei B21060 plus arabinogalactan and xilooligosaccharides in children with acute diarrhoea. Aliment Pharmacol Ther. 2012 Apr;35(7):782–8.
132. SzymaÅ„ski H, Pejcz J, JawieÅ„ M, Chmielarczyk A, Strus M, Heczko PB. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains—a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006 Jan 15;23(2):247–53.
133. Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007 Aug 18;335(7615):340.
134. Chen K, Xin J, Zhang G, Xie H, Luo L, Yuan S, et al. A combination of three probiotic strains for treatment of acute diarrhoea in hospitalised children: an open label, randomised controlled trial. Benef Microbes. 2020 Aug 12;11(4):339–46.
135. Szajewska H, Canani RB, Guarino A, Hojsak I, Indrio F, Kolacek S, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016 Mar;62(3):495–506.
136. Szajewska H, KoÅ‚odziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015 Nov;42(10):1149–57.
137. Lukasik J, Dierikx T, Besseling-van der Vaart I, de Meij T, Szajewska H, Multispecies Probiotic in AAD Study Group. Multispecies probiotic for the prevention of antibiotic-associated diarrhea in children: a randomized clinical trial. JAMA Pediatr. 2022 Sep 1;176(9):860–6.
138. RuszczyÅ„ski M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther. 2008 Jul;28(1):154–61.
139. Szajewska H, Wanke M, Patro B. Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment Pharmacol Ther. 2011 Nov;34(9):1079–87.
140. Hojsak I, Szajewska H, Canani RB, Guarino A, Indrio F, Kolacek S, et al. Probiotics for the prevention of nosocomial diarrhea in children. J Pediatr Gastroenterol Nutr. 2018 Jan;66(1):3–9.
141. Beghetti I, Panizza D, Lenzi J, Gori D, Martini S, Corvaglia L, et al. Probiotics for preventing necrotizing enterocolitis in preterm infants: a network meta-analysis. Nutrients. 2021 Jan 9;13(1):192.
142. Chi C, Li C, Buys N, Wang W, Yin C, Sun J. Effects of probiotics in preterm infants: a network meta-analysis. Pediatrics. 2021 Jan;147(1):e20200706.
143. Gao X, Wang Y, Shi L, Feng W, Yi K. Effect and safety of Saccharomyces boulardii for neonatal necrotizing enterocolitis in pre-term infants: a systematic review and meta-analysis. J Trop Pediatr. 2021 Jul 2;67(3):fmaa022.
144. van den Akker CHP, van Goudoever JB, Szajewska H, Embleton ND, Hojsak I, Reid D, et al. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J Pediatr Gastroenterol Nutr. 2018 Jul;67(1):103–22.
145. Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: a strain-specific systematic review. JPEN J Parenter Enteral Nutr. 2016 Aug;40(6):783–94.
146. Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005 Jan;115(1):1–4.
147. Feng JR, Wang F, Qiu X, McFarland LV, Chen PF, Zhou R, et al. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol. 2017 Oct;73(10):1199–208.
148. Wen J, Peng P, Chen P, Zeng L, Pan Q, Wei W, et al. Probiotics in 14-day triple therapy for Asian pediatric patients with Helicobacter pylori infection: a network meta-analysis. Oncotarget. 2017 Nov 10;8(56):96409–18.
149. Zhou BG, Chen LX, Li B, Wan LY, Ai YW. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: a systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019 Oct;24(5):e12651.
150. Szajewska H, Horvath A, KoÅ‚odziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2015 Jun;41(12):1237–45.
151. Fang HR, Zhang GQ, Cheng JY, Li ZY. Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials. Eur J Pediatr. 2019 Jan;178(1):7–16.
152. Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009 Jan;98(1):127–31.
153. Bin Z, Ya-Zheng X, Zhao-Hui D, Bo C, Li-Rong J, Vandenplas Y. The efficacy of Saccharomyces boulardii CNCM I-745 in addition to standard Helicobacter pylori eradication treatment in children. Pediatr Gastroenterol Hepatol Nutr. 2015 Mar;18(1):17–22.
154. Viazis N, Argyriou K, Kotzampassi K, Christodoulou DK, Apostolopoulos P, Georgopoulos SD, et al. A four-probiotics regimen combined with a standard Helicobacter pylori-eradication treatment reduces side effects and increases eradication rates. Nutrients. 2022 Feb 1;14(3):632.
155. Sung V, D’Amico F, Cabana MD, Chau K, Koren G, Savino F, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018 Jan;141(1):e20171811.
156. Skonieczna-Å»ydecka K, Janda K, Kaczmarczyk M, Marlicz W, Å