Need for Endoscopic Interventions and a Multimodality Approach in the Era of Living Donor Liver Transplantation in South Korea
Vol. 28, Issue 3 (September 2023)
 |
DongKi Lee, MD, PhD
Department of Internal Medicine
Gangnam Severance Hospital
Yonsei University
Seoul, Korea
|
| |
|
Liver Transplantation in South Korea
Liver transplantation (LT) in South Korea differs markedly from that in Western countries in terms of etiology and method. The rate of living donor liver transplantation (LDLT) is more than twofold higher than that of deceased-donor LT (DDLT). South Korea has the largest number of living donors per million people among Asia–Pacific countries and ranks second worldwide in terms of the number of living donors per million people (IRODaT 2019). Since the performance of the first LDLT in South Korea in 1997, the rate of LDLT has steadily increased. South Korea is the world leader in LDLT, with the largest annual number of transplantation surgeries, 22.9 per million people. This is more than tenfold that in Japan and Hong Kong, which have the highest frequencies of LT worldwide (IROaT 2019).1 Due to the shortage of brain-death donors in South Korea, DDLT is performed in patients with a high MELD score. In South Korea’s family-centered culture, this surgery has become a source of hope for patients with end-stage liver disease.2
South Korea has developed innovative surgical techniques, which are now the standards at most centers worldwide. South Korean physicians established right-lobe LDT some years ago. LDLT is now commonly performed in South Korea for the treatment of hepatocellular cancer and end-stage liver cirrhosis.
Biliary complications after LDLT differ from those after DDLT
Biliary stricture and bile leak are the primary targets for endoscopic and percutaneous interventions. Biliary complications are the most common complications after DDLT and LDLT.
Biliary Stricture
LDLT is technically more complex and challenging than DDLT and has a higher incidence of biliary complications. Bile duct strictures are the most common biliary complication after LT, accounting for approximately 40% of all biliary complications. Bile duct stricture reportedly occurs in up to 5% of DDLT cases, compared to 7.3–60% for right-lobe grafts and 24% for left-lateral-segment grafts for LDLT.
In recent years, duct-to-duct reconstruction has been preferred over hepaticojejunostomy in LDLT, because of its simplicity, rapid gastrointestinal recovery, and preservation of physiological biloenteric continuity. A duct-to-duct anastomosis can be easily approached endoscopically, mostly by ERCP, rather than hepaticojejunostomy. However, when this is performed, the site is higher (at the hilum) than in DDLT. In LDLT, the angle between the bile duct of the received liver and the extrahepatic bile duct is acute, which is associated with a risk for ischemia and traction in surrounding tissues. As the transplanted liver becomes hypertrophic, an anastomotic stricture is possible. A post-LT anastomotic biliary stricture (ABS) is typically caused by an improper surgical technique, including excessive use of electrocoagulation, tension at the level of the anastomosis, and inappropriate bile duct dissection, as well as by small-caliber bile ducts, localized ischemia, infection, or fibrotic healing, with most cases occurring within 12 months after LT. The ABS after LDLT is more frequent and challenging than DDLT for the above reasons.3
Non-anastomotic strictures (NAS) typically result from hepatic artery injury or thrombus, causing irreversible biliary fibrosis as a result of ischemia. Other causes include prolonged cold ischemia or ABO-type incompatibility.
Bile Leak
Bile leak is the second most common biliary adverse event following LT and results in significant morbidity among LT recipients. A bile leak is a risk factor for biliary strictures and vice versa. Bile leaks occur in up to 25% of LT cases, typically one day to six months after the operation. The leak can occur from the anastomosis, biliary drainage tube tract, liver cut surface, or cystic duct remnant. Anastomotic leak is the most common type. The frequency of bile leak is 7.5% after DDLT and 9.5% after LDLT.4
Endoscopic management is the initial treatment modality for biliary complications
Biliary Stricture
The mainstay of ABS and NAS management is ERCP therapy. Most patients require several ERCP sessions at three-month intervals, with stenting and dilation for more than one year. Plastic stents should be exchanged regularly to avoid occlusion-causing cholangitis. The stricture resolution rate for patients with ABS treated with plastic stents via ERCP is 80%. The rate of recurrence depends on the duration of stenting; less than one year of stenting has a 78% stricture resolution rate, compared to 97% for more than one year.
There have been attempts to overcome the limitations of periodic plastic stent replacements using temporary single-session self-expanding metal stents (SEMSs).5, 6 However, because SEMSs have high migration rates and variable results, they do not yield a consistently superior resolution of ABS compared to maximal plastic stent therapy. In South Korea, for the treatment of proximal benign biliary stricture, a modified fully covered SEMS is used, which has a mid-waist to prevent migration and a long string to facilitate removal using standard endoscopic grasping forceps. The waist, at the central portion, prevents migration of the stent. The stent is available at diameters of 6.8 and 10 mm and lengths of 4, 5, and 6 cm, enabling clinical use according to the anatomical situation. The short removable fully covered SEMS is an effective treatment for ABS after LT that is not amenable to treatment by conventional procedures with plastic stents (Fig. 1). In addition, a plastic stent should be inserted if obstruction of a branch of the intrahepatic bile duct by a fully covered SEMS is suspected. The fully covered SEMS is a novel salvage treatment option for ABS after LDLT.
Dilation of NAS typically requires a smaller balloon than ABS. The efficacy of ERCP or percutaneous treatment for NAS is less than that for ABS, and NASs require a longer duration of treatment. In addition, there is a higher rate of stent failure due to migration or occlusion. NAS strictures that occur in the intrahepatic region of the biliary tree are difficult to access endoscopically.
Bile Leak
The most widely accepted treatment in patients with duct-to-duct biliary anastomosis is early ERCP-guided endoscopic therapy. Usually, a sphincterotomy is performed, and a transpapillary stent is placed for two to three months to divert bile from the leak. This decreases the transpapillary pressure gradient, which can exacerbate bile leaks. A longer duration of stent placement than in cholecystectomy cases are recommended because of delayed healing in the setting of immunosuppression. The fully covered SEMS can effectively treat refractory or bile leaks with a large defect.
Given the anatomy, bile leaks after Roux-en-Y in LDLT are rarer and difficult to treat by ERCP. If unable to obtain biliary access endoscopically, a percutaneous internal-external drainage can be performed to treat bile leaks. Surgery will be necessary if these measures are unsuccessful.
Treatment of complex complications after LDLT requires a multimodality approach
Advantages and Need for Collaboration between Percutaneous and Endoscopic Approaches Under Challenging Cases
In terms of treating biliary strictures, the donor bile duct and narrowing are smaller and more anatomically challenging in LDLT. These features are more prominent in LDLT than in DDLT patients. ERCP is a feasible first treatment modality for post-LDLT biliary stricture, but in failed cases, particularly with a pouched anastomosis, percutaneous transhepatic biliary drainage (PTBD) can be attempted. Stricture type is a determinant of successful guidewire passage rather than the procedure equipment or procedure time. Among the pouched, triangular, and intermediate forms of the distal side of the bile duct anastomosis, the pouched form has the lowest endoscopic success rate (25%).7 If contrast medium readily passes into the intrahepatic duct in a pouched-form case, we spend more time than typical attempting to pass the guidewire. Spending too much time on ERCP in patients with a pouched-form anastomosis is undesirable. If the results fall short of expectations, we convert to a percutaneous approach (Fig. 2).
The success rate of PTBD for resolving a bile duct anastomosis stricture in pouched-form cases is 60% on the first attempt, but the total success rate, including repeated PTBD, is 87%. Therefore, PTBD is useful for cases of failed endoscopic therapy for a post-LDLT biliary stricture. We attempt a second PTBD because we believe that the major reason for the failure of the first PTBD is not the tightness of the anastomotic stricture area but a tortuous and kinked anastomotic stricture because of the compression effect of the transplanted liver. To pass a guidewire through a stricture, PTBD is more effective than ERCP, because torque can be applied on a short guidewire using a variably shaped pathfinding catheter during PTBD. In addition, PTBD has advantages over ERCP in terms of patient comfort (e.g., being in the supine position during the procedure) and offers a more exact biliary anatomy. If PTBD succeeds, one may exchange the PTBD catheter for an internal stent using the rendezvous method.
Cooperation with intervention radiology is frequently necessary when treating complicated or refractory bile leaks after LDLT. Additional percutaneous stenting is mandatory if the biliary defect is large and per-oral, stenting cannot effectively bridge the bile leak. If the per-oral approach fails to enable selective cannulation of the leak area, the percutaneous method is typically effective (Fig. 3), e.g., for bile leaks in patients with multiple anastomoses of the bile ducts.
Magnet Compression Anastomosis for the Treatment of Total Biliary Obstruction
Endoscopic and percutaneous procedures have high success rates in post-LT ABS. However, recanalization is impossible using conventional endoscopic and percutaneous methods in cases of a severe stricture or complete obstruction that prevents the passage of the contrast medium and guidewire. In such cases, surgical revision must be performed or external drainage catheters must be maintained. Surgical revision of BBSs is associated with high morbidity and mortality rates. Moreover, there is a high rate of recurrent strictures requiring further interventions following surgical revision. Catheter-related complications can arise when percutaneous external drainage catheters are maintained, compromising the patient’s quality of life.
Magnet compression anastomosis (MCA) was developed as a nonsurgical alternative for patients with BBSs in whom conventional endoscopic or percutaneous methods failed (Fig. 4). 8, 9, 10 The attractive force from the two magnets on both sides of the ABS creates compression, which induces ischemia in the ABS tissue. As the two magnets approach each other, complete necrosis of ABS tissues occurs, and a new fistula is formed to complete the recanalization. MCA is safe and in animal studies has been found to be equivalent or superior to anastomoses created by traditional suturing or stapling techniques. Our institution has performed MCA on more than 70 patients after LDLT, with a success rate of more than 90% and a low rate of stricture recurrence. Two magnet-delivery routes are needed to perform this procedure, typically one PTBD and one ERCP. An appropriate percutaneous magnet delivery route is key for procedure success, which makes cooperation with intervention radiology essential.
Conclusion
In South Korea, there are more biliary complications after LDLT than after DDLT, with biliary stricture being the most common. ERCP is a feasible initial treatment modality for post-LDLT complications, and PTBD can be used in failed cases.
In the future, most LTs will be LDLT; DDLT is becoming rare worldwide. As experience with LDLT accumulates and the surgical technique improves, the incidence of biliary tract complications after LDLT may decrease. However, after LDLT one or more biliary complications is inevitable, the treatment of which—particularly of complicated and refractory biliary complications—requires a multimodality approach.11
Figure legend
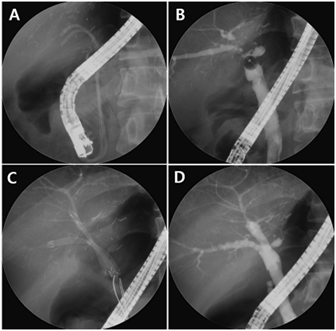
Figure 1. Fully covered self-expandable metal stent (FCSEMS) for the treatment of refractory anastomotic biliary stricture
(A) The patient was referred to our hospital with two plastic stents. (B) Cholangiogram showing multiple tight anastomotic strictures in the posterior and inferior intrahepatic ducts. (C) FCSEMSs were sequentially inserted into the stricture sites. (D) After an indwelling time of 3 months, the retrieval strings were grasped using biopsy forceps to remove the FCSEMSs. The cholangiogram demonstrates the resolution of multiple strictures.
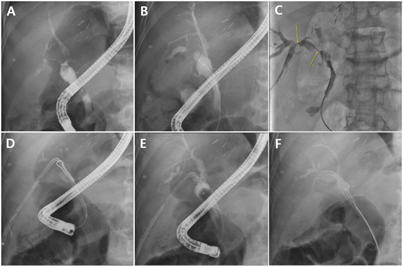
Figure 2. Percutaneous treatment of pouch-type anastomotic biliary stricture (ABS) after living donor liver transplantation
(A) ERCP cholangiography showing pouch-type ABS. (B) Guidewire passing through the pouch-type ABS; ERCP was attempted but failed. Only the right posterior duct was successfully cannulated. (C) Guidewire was successfully passed through the ABSs (yellow arrows) using the percutaneous approach. Percutaneous transhepatic biliary drainage (PTBD) catheter was placed in the ABS. (D) Successful guidewire insertion with ERCP in the pouch-type ABS using the rendezvous method. (E) After passing the guidewire through the ABS, the PTBD catheter was removed. (F) Fully covered self-expandable metal stent (FCSEMS) was inserted into the ABS, and a plastic stent was placed in the right posterior duct to prevent bile duct obstruction by the FCSEMS membrane.
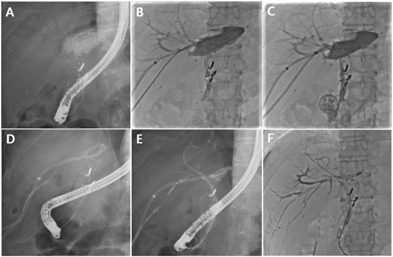
Figure 3. Percutaneous bile leakage treatment after living donor liver transplantation (LDLT)
(A) Cholangiogram showing bile leakage after LDLT. Selective cannulation of the anastomosis site with ERCP was not possible. (B) A percutaneous catheter was inserted to drain the leaked bile. Another PTBD catheter at B6 was used to pass a guidewire through the common bile duct (CBD). Selective cannulation of the CBD using the guidewire was successful. (C) The percutaneous transhepatic biliary drainage (PTBD) catheter remained indwelling across the bile leak area. (D) A guidewire was successfully passed with ERCP in the leaking bile duct 1 month later using the rendezvous method. (E) Fully covered self-expandable metal stent (FCSEMS) was inserted into the leaking bile duct, and a plastic stent was placed to prevent blockage by the stent membrane. (F) After FCSEMS insertion, the amount of leaked bile decreased, and the percutaneous drainage catheter could be removed.
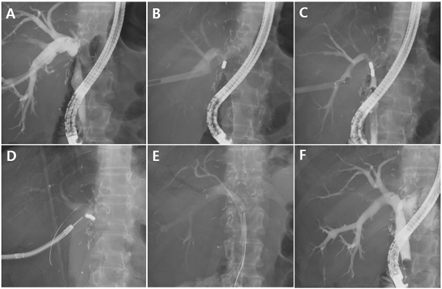
Figure 4. Magnetic compression anastomosis in a benign biliary stricture patient after living donor liver transplantation
(A) Percutaneous transhepatic biliary drainage (PTBD) catheter was inserted and dilated up to 16 Fr. We preferred an indwelling fully covered self-expandable metal stent (FCSEMS) at the ampulla because of the ease of magnet delivery in ERCP. (B) A magnet grasped with a polypectomy snare was delivered into the common bile duct (CBD) with ERCP. (C) The proximal magnet was delivered through the 18 Fr. sheath PTBD tract. Magnet alignment could be confirmed during the procedure. (D) The approximated magnets were removed after for weeks using a percutaneous transhepatic cholangioscopy. (E) After magnet removal, the indwelling FCSEMS remained at the new fistulous tract for six months. (F) The FCSEMS was removed and fistula formation was evident.
References
1.“Transplantation,” Medical Korea, accessed 2023.04.10., https://www.medicalkorea.or.kr/en/Transplantation.
2. Choi HJ. Current status and outcome of liver transplantation in south Korea. Clin Mol Hepatol. 2022;28:117-119.
3. Jang SI, Lee DK. Biliary complications after living donor liver transplantation differ from those after deceased donor liver transplantation.Gut and Liver 2022;16:145-146
4. Rao HB, Prakash A, Sudhindran S,Veny RP. Biliary strictures complicating living donor liver transplantation: Problems, novel insights, and solution. World J Gastroenterol
2018;21:2061-2072.
5. Jang SI, Chung TR, Cho JH et al. Short fully covered self-expandable metal stent for treatment of proximal anastomotic benign biliary stricture after living-donor liver transplantation. Dig Endosc
2021;33:840-848.
6. Jang SI, Sung SY, Park H, Lee K-H, Joo S-M, Lee DK. Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after living-donor liver transplantation. Ther Adv
Gatroenterol 2017;10;297-309.
7. Kim ES, Lee BJ, Won JY, Choi JY, Lee DK. Percutaneous transhepatic biliary drainage may serve as a successful rescue procedure in failed cases of endoscopic therapy for a post-living donor liver transplantation biliary stricture. Gastrointest Endosc 2009;69:38-46.
8. Jang SI, Kim J-H, Won JY, et al. Magnetic compression anastomosis is useful in biliary anastomotic strictures after living donor liver transplantation. Gastrointest Endosc-
2011;74:1040-1048.
9. Jang SI, Cho JH, Lee DK. Magnetic compression anastomosis for the treatment of post-transplantation biliary stricture. Clin Endosc- 2022;53:266-275.
10. Han SJ, Jang SI, You SH, Lee DK. A case with combined postoperative bile leakage and anastomotic stricture after liver transplantation treated with magnet compression anastomosis. Int J Gastrointest
Interv 2022;9:31-33.
11. Jang SI, Lee DK. Anastomotic stricture after liver transplantation: it is not Achilles’ heel anymore! Gatrointest Interv 2018;7:57-66.






